A First in Class Product that can act as a “Platform” Applicable to Multiple Diseases
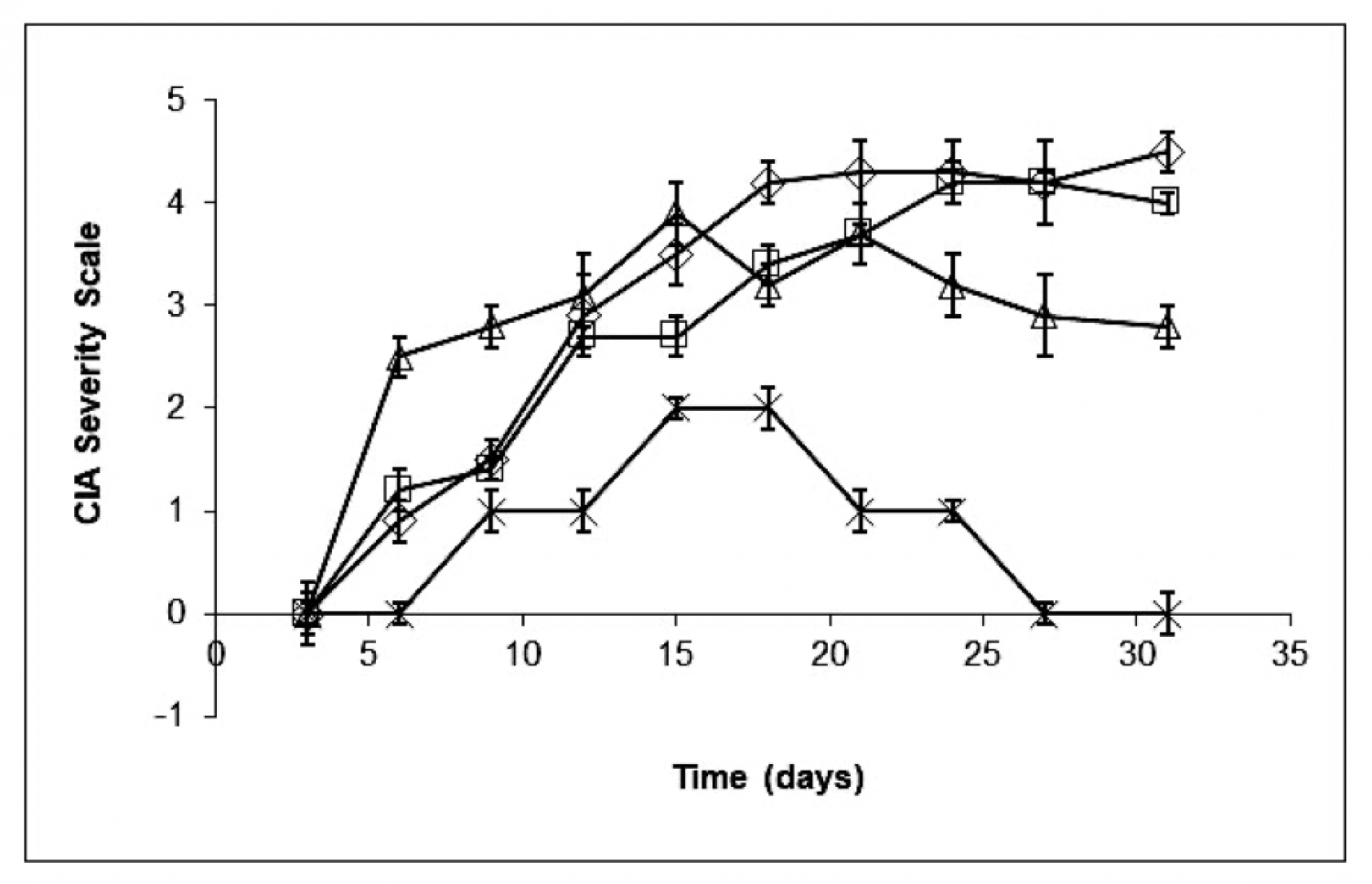
12 mice where used per group. ImmCelz® was addadministered to mice 14 days after last collagen injection. Control ( diamond ) mice had rapid onset of disease. 100,000 inactive ImmCelz® cells (square). Administration of 100,000 active ImmCelz® cells (X) resulted in disease protection. Depletion of T regulatory cells by removal of CD25 positive cells resulted in negation of protection (triangle).
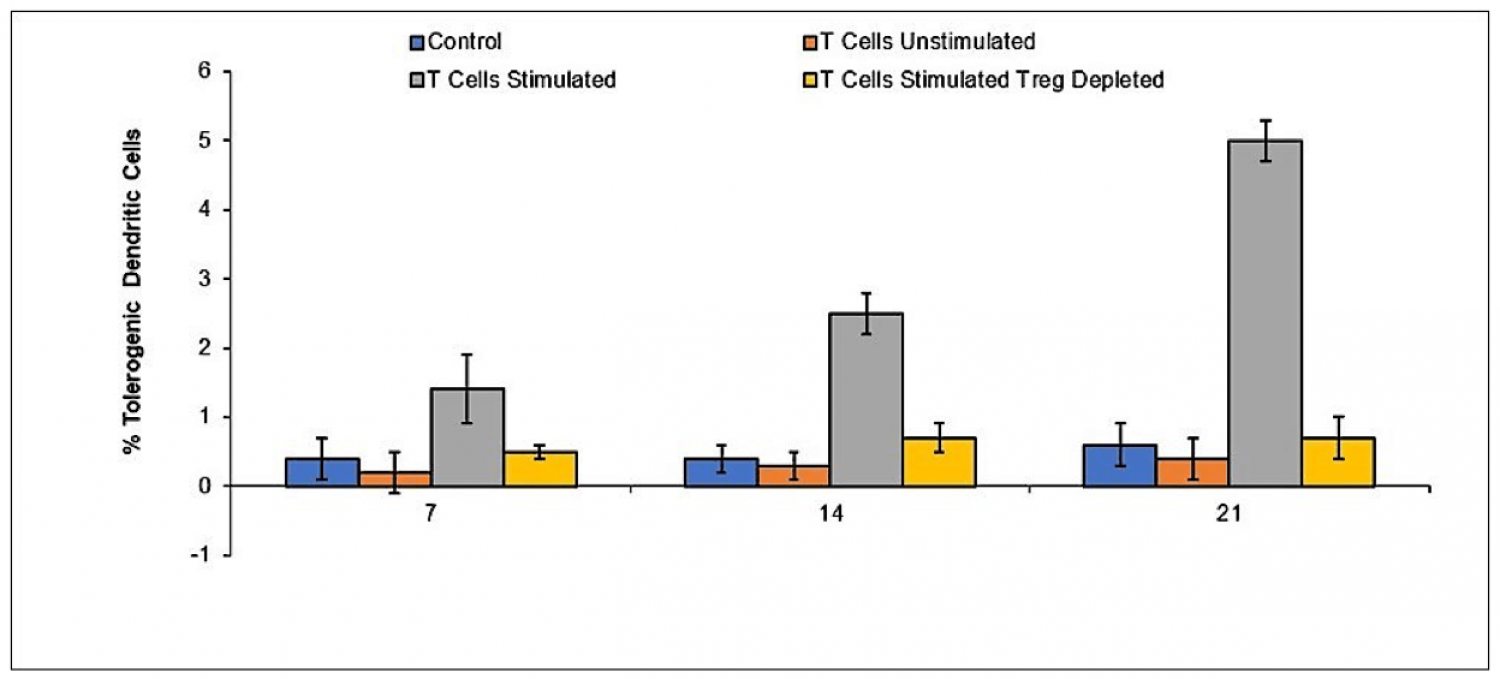
Allogeneic peripheral blood mononuclear cells (PBMC) were incubated with umbilical cord mesenchymal stem cells for 48 hours in a transwell chamber and subsequently added to lipopolysaccharide stimulated macrophages at a 1:1 ratio. Assessment of TNF-alpha was performed by ELISA at the indicated time points